
Savannah Conlon
Education:
B.S. Chemistry, Minor in Biology, University of California, Santa Cruz, 2017
From: Grass Valley, California
Joined David Lab: January 2018
Outside of lab: I enjoy long runs and skiing. You can catch me in the kitchen several nights a week because I love cooking!
Research in David Lab:
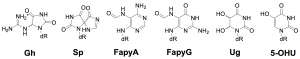
Oxidative damage to our DNA by endogenous or exogenous sources must be repaired to maintain the integrity of the genome. This type of damage is repaired via the base excision repair pathway, where glycosylases recognize and initiate repair. Specifically, I study the NEIL family of DNA glycosylases, which are required for the removal of oxidized bases from DNA. One of these enzymes, NEIL1, has been heavily characterized by our laboratory. NEIL1 has the ability to recognize and cleave a variety of oxidized lesions from differing DNA contexts. Previous work has shown that there are two wild type isoforms of this enzyme due to pre-mRNA editing by ADAR1. The two forms differ at one amino acid position, yielding either lysine or arginine at position 242 of the enzyme. Each form has different substrate preferences, some of which are shown below; therefore, it is of particular interest to investigate other differences between them. I am continuing to evaluate the intrinsic rate of glyosidic bond cleavage and the binding affinity of each form of NEIL1 on different lesions, and plan to continue this investigation with NEIL3. After seeing such a distinct difference between the two isoforms of NEIL1 in vitro, I am also interested in comparing repair initiated by each isoform in cellular assays. I want to determine if the same trends that occur in vitro also exist in a cellular context. My hope is to further elucidate the cellular benefit of having two such isoforms.
Previous Research Experience:
I worked in Dr. Scott Lokey’s lab at UC Santa Cruz as an undergraduate, where I studied cyclic peptides inspired by natural products. Specifically, I worked on optimizing peptide macrocyclization by studying the effect of various reaction conditions. I aimed to help determine how to limit the unwanted oligomerization of peptides during cyclization.
